Research |
The Barnes research group works at the interface of chemistry, engineering, materials science, biology, and medicine. The overarching goal of our research is to develop next-generation polymeric materials that we as synthetic chemists can program at the (macro)molecular level with precise functions, resulting in advanced materials that have enhanced properties for a broad range of applications. Specifically, we design functional polymers and crosslinkers with non-covalent bonding capabilities – i.e., supramolecular polymers – that adopt unique pathways of self-assembly and/or activation. Thus, we create functional materials from the nanometer to the macroscopic scale and are inspired by Nature’s forms of molecular recognition and function, but possess the robustness and enhanced durability of synthetic materials. Below, you can find more information about the four main project areas in the Barnes group: (1) photoredox-responsive covalent hydrogels; (2) the use of ROMP to make nanoparticles and hydrogels for drug delivery and energy storage; (3) the synthesis of mechanically interlocked molecules to control entanglement in polymer networks; and (4) a new project on establishing new methodologies to synthesize and fabricate high-performance fiber-based materials.
(1) New (Photo)Redox-Responsive Polymers and Mechanisms for Actuating Soft Materials
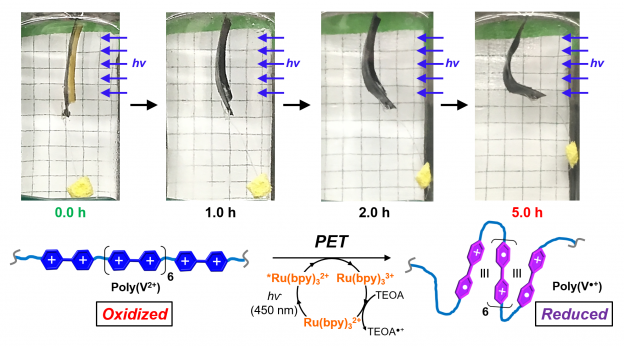
The Barnes group is interested in the design, synthesis, and function of redox-responsive macromolecules and macrocrosslinkers, where the primary functional component is based on viologens (4,4'-bipyridinium). We discovered that by integrating oligo- and polyviologens into hydrogel polymer networks, it is possible to reversibly contract macroscopic materials through a concerted mechanism that involves reduction of a dicationic viologen (V(2+)) to its radical cation (V(•+)), which induces viologen radical-based self-assembly (i.e., chain folding), while also decreasing the number of positive charges by half and losing a corresponding number of counteranions. We have also shown that it is possible to reverse this contraction mechanism simply by oxidizing the viologen subunits back to their dicationic oxidation state in the presence of ambient O2 and re-swelling the hydrogels in water. We also demonstrated that a photoredox catalytic cycle could be implemented to reduce the polyviologens in situ, which affords spatiotemporal control over the actuation using visible light. We are now exploring these materials to improve actuator properties and performance for a variety of applications.
(1) New (Photo)Redox-Responsive Polymers and Mechanisms for Actuating Soft Materials
The Barnes group is interested in the design, synthesis, and function of redox-responsive macromolecules and macrocrosslinkers, where the primary functional component is based on viologens (4,4'-bipyridinium). We discovered that by integrating oligo- and polyviologens into hydrogel polymer networks, it is possible to reversibly contract macroscopic materials through a concerted mechanism that involves reduction of a dicationic viologen (V(2+)) to its radical cation (V(•+)), which induces viologen radical-based self-assembly (i.e., chain folding), while also decreasing the number of positive charges by half and losing a corresponding number of counteranions. We have also shown that it is possible to reverse this contraction mechanism simply by oxidizing the viologen subunits back to their dicationic oxidation state in the presence of ambient O2 and re-swelling the hydrogels in water. We also demonstrated that a photoredox catalytic cycle could be implemented to reduce the polyviologens in situ, which affords spatiotemporal control over the actuation using visible light. We are now exploring these materials to improve actuator properties and performance for a variety of applications.
(2) Ring-Opening Metathesis Polymerization to Construct Soft Materials from Self-Assembled Polymer Architectures
We employ ring-opening metathesis polymerization (ROMP) to synthesize functional architectures that can be used for applications ranging from drug delivery to energy storage. Current collaborations through WashU's School of Medicine focus on developing nanoparticles that can deliver therapeutic cargo to treat brain cancer. Other research endeavors entail using visible and near-IR light to accelerate the release of negatively charged antibiotics from positively charged viologen units inside the network of covalent or self-assembled hydrogels. We are also collaborating with groups in Engineering at WashU to develop new high-performance materials for rechargeable batteries.
We employ ring-opening metathesis polymerization (ROMP) to synthesize functional architectures that can be used for applications ranging from drug delivery to energy storage. Current collaborations through WashU's School of Medicine focus on developing nanoparticles that can deliver therapeutic cargo to treat brain cancer. Other research endeavors entail using visible and near-IR light to accelerate the release of negatively charged antibiotics from positively charged viologen units inside the network of covalent or self-assembled hydrogels. We are also collaborating with groups in Engineering at WashU to develop new high-performance materials for rechargeable batteries.
(3) Topologically Complex Molecules, Polymers, and Materials
Entanglement in polymer networks usually occurs randomly yet it plays a significant role in the properties of polymeric materials. Over the years, chemists have become adept at making mechanically interlocking molecules (MIMs) from physically (as opposed to covalently) connected macrocycles, representing a controlled method for generating entangled molecular architectures. Our research group is interested in improving the synthetic methodologies required to make MIMs, particularly multi-macrocycle structures that resemble real-world chains (technically referred to as [n]catenanes), such that we can then investigate them in the context of polymers and polymer networks. Using metal-based templation and ring-closing metathesis, we have shown that it is possible to interlock up to eight macrocycles in a discrete linear strand, and that catenane-based crosslinkers in gels and thermosets can be tuned via metalation/demetalation to control the molecular motions of the entangled rings.
Entanglement in polymer networks usually occurs randomly yet it plays a significant role in the properties of polymeric materials. Over the years, chemists have become adept at making mechanically interlocking molecules (MIMs) from physically (as opposed to covalently) connected macrocycles, representing a controlled method for generating entangled molecular architectures. Our research group is interested in improving the synthetic methodologies required to make MIMs, particularly multi-macrocycle structures that resemble real-world chains (technically referred to as [n]catenanes), such that we can then investigate them in the context of polymers and polymer networks. Using metal-based templation and ring-closing metathesis, we have shown that it is possible to interlock up to eight macrocycles in a discrete linear strand, and that catenane-based crosslinkers in gels and thermosets can be tuned via metalation/demetalation to control the molecular motions of the entangled rings.
(4) Synthesis and Fabrication of High-Performance Fibers
Brand New Project Alert! More info coming soon...
Brand New Project Alert! More info coming soon...